
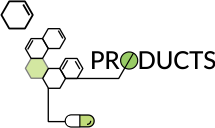
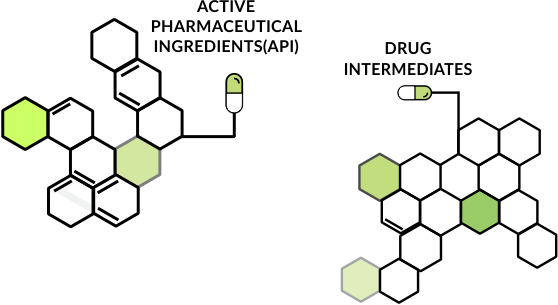
ANLON Healthcare is a research-intensive manufacturing unit of API and its intermediates. Based in Rajkot, we've earned a global recognition for manufacturing products that adhere to the highest standards of quality. Our products comply with the regulatory requirements of leading health authorities such as FDA, PMDA, KFDA, cGMP, WHO-GMP. With exceptional R&D capabilities, advanced API manufacturing facilities, global regulatory accreditations, a strong product pipeline and speed-to-market competencies, ANLON is an ideal API partner.
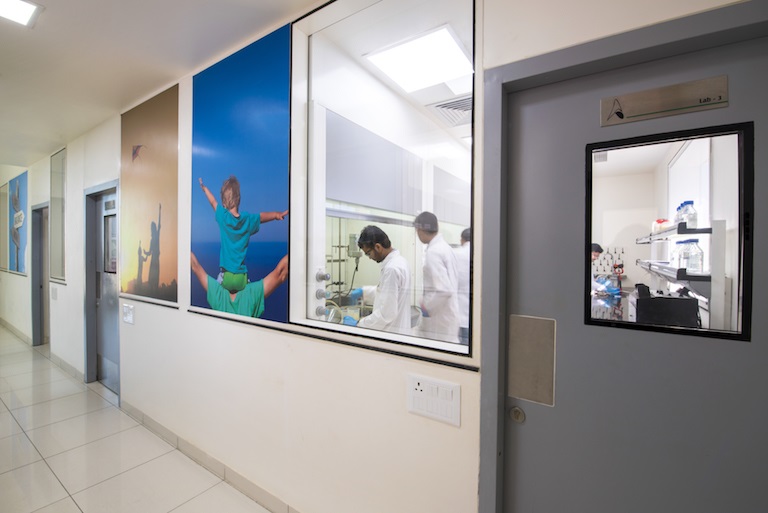
We offer thorough technical and regulatory support across the entire production process, from project initiation to final production. Our manufacturing facility is equipped with high precision instruments that build quality at every stage of the process. We have experts at the helm of our organization, dedicated to safeguarding the quality and class of our products.
Owing to the emerging competitive climate of the pharmaceutical industry, we've been proactive in discerning ways to continually add customer value and augment our competency in business. This means that our products are continually upgraded to keep pace with the evolving dynamics of the global environment. With the range of high quality API and Advanced Drug Intermediates, ANLON has been successful in carving out a competitive niche in the market.
OUR MISSION
With an approach to innovation that is compelling, inclusive and visionary, we seek to drive efficiency and productivity while delivering tangible results.
OUR VISION
To harness the strength of technology and research into becoming a leading global pharmaceutical company that delivers maximum consumer satisfaction.

- ANLON Healthcare has the expertise and infrastructure available that creates a conducive work environment that inspires innovation at all levels. An impeccable adherence to quality and an uncompromising will to deliver promises, is what ANLON stands for.
- Our ultramodern manufacturing facilities are cGMP compliant in accordance with various national and international standards. Acuity and precision are guaranteed owing to the high-tech, sophisticated instruments.
- The Research & Development division is an indispensable asset to our company. It is not only aimed at improving the performance and efficacy of the existing products, but also developing new products that address the ever evolving needs of the market.
- The cornerstone of our successful journey, rests undoubtedly, in the brain power of our highly skilled and dedicated employees. They are unrelenting in their commitment to ensuring that the highest quality is built into our products.
- Quality consciousness is made to percolate in every stage of the production process. We are committed to providing the best-in-class drug development solutions that are cost-effective, sustainable and reliable.

SYNERGY
At ANLON we believe that customer satisfaction is a key metric to project management success. We customize our approach to cater to the needs of our clients and advocate their aspirations as our own. We align our incentives with their objectives and help them unleash their maximum potential by engaging their efforts with the best of what is available in the market. With its synergistic strengths and unique innovation, ANLON has been unrelenting in its pursuit for excellence and consistency of service.
STRATEGY
Our only strategy is innovation. Relentless and continual. By wielding technology to propel progress, we adapt and evolve with the growing needs of the market. Our persistent quest for novelty and enhancement in competence ensures that our customers get what they want, need and expect.
SUSTAINABILITY
At ANLON, we are committed to meeting high ethical standards thereby creating and maintaining a healthy environment for all. We measure our achievements at three levels- satisfaction of our customers, efficiency of our employees and finally, impacting change in the society. Our commitment to social responsibility is embedded in our core values - integrity, accountability and authenticity.

















































We are a team of impassioned technocrats who believe in the remarkable power of synergism and collaboration. Together we question assumptions, surpass limitations and exceed expectations. And to further augment our competence and go the extra mile, we’re hiring! Here is an opportunity for you to experience and contribute to the building of a company, its culture and to put your skills at work; a chance to reap personal and professional benefits; a chance to work with the best of what is available in the market. With its innovation-led growth strategy, ANLON is a revolution that has taken the pharmaceutical industry by storm and is looking for trailblazers with an unquenchable thirst for curiosity, to be a part of its amazing team. Discover our business, understand our culture and join us on our way to becoming a leading pharmaceutical company that people can rely on.
APPLY NOW

ANLON HEALTHCARE PVT. LTD.
OFFICE : 101/102-Silver Coin Complex,
Opp. Crystal Mall, Kalawad Road,
Rajkot 360005,Gujarat (INDIA)
Phone: 281-2561340, 2562538/39
FACTORY : Anlon Healthcare Pvt. Ltd.,
Survey No. 36/2, Near Bharudi Toll Plaza,
Gondal Road NH27,
PO: Pipaliya (Sadak), Tal: Gondal, Rajkot, (INDIA)
Phone:+91-7069690081/82

